Chapter 5b
Chemistry of Seawater
Seawater Is a Chemical Solution
Solvent – the water is the
dissolving agent
Solute – the salt ions (and other
compounds) dissolved in the water
Concentrations of dissolved
salts are
measured in parts per thousand (o/oo)
Major ions in seawater
Six most abundant ions
account for over 99% of the solutes in seawater
chloride Cl–
sodium Na+
sulfate SO4–2
magnesium Mg+2
calcium Ca+2
potassium K+
bicarbonate HCO3–
Conservative Ions
The major ions in seawater
vary little over time and location in the ocean
They are not affected by
biological activity
Linear mixing between water masses with
different salinities
Other Dissolved Constituents
Nutrients – needed for phytoplankton
growth
N (as nitrate) P (as phosphate) Si
concentrations in the ocean in
parts per million (ppm) or milligrams per liter (mg / L)
Gases – surface water in
equilibrium with the atmosphere
N2 O2 CO2 H2 noble gases: Ar Ne
He
Trace elements – practically all natural
elements are dissolved in seawater
but in parts per billion (ppb) or parts per trillion
** life is adapted to
extremely low concentrations of these elements,
even slight contamination may be toxic **
Organic compounds – large, complex molecules
produced by organisms
Principle of Constant Proportion
The ratio between the major ions is constant,
regardless of the salinity
So, the ratio of Na+ / K+ is the same
at 5 ‰ at the head of an estuary
at 25 ‰ near the mouth of an estuary
at 30 ‰ on the continental shelf
at 35 ‰ in the open ocean
Salinity from Chlorinity
Salinity is the total weight in
grams of salts dissolved in 1 kilogram of seawater
Salinity can be calculated by measuring chlorinity
(all of the halides)
salinity (‰) = 1.80655 x chlorinity (‰)
Salinity analyses in the lab
Titration – chemical reaction of
chloride with silver nitrate
Hygrometer – measures density
Salinometer – measures conductivity
Practical Measurements of Salinity
In practice, salinity is measured with a precisely calibrated salinometer
which measures the electrical conductivity of seawater, and
corrects for the temperature
practical salinity units (psu)
Water sampling at sea
CTD: conductivity temperature depth
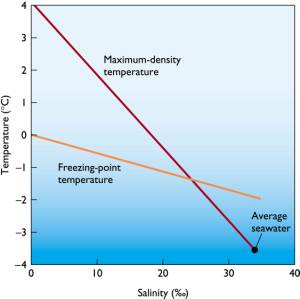
Effect of Salinity
Salinity
depresses freezing point and temperature of maximum density
The Clines
Thermocline – a sharp change in
temperature with water depth
Halocline – a sharp change in
salinity with depth
Pycnocline – a sharp change in
density with depth
Thermocline by latitude
Near
the poles, almost uniform temperatures through the water column,
allows
sinking of cold surface water
Global sea-surface temperature
Several abrupt changes in
surface temperature – for example, the polar front
Latitudinal temperature section – Pacific
Most pronounced thermocline
in low latitudes
Seasonal thermocline – warming
Stratification of the water
column as solar heating increases in summer
Seasonal thermocline – cooling
Destratification and mixing
(turn-over) as surface water cools in the fall
Balance of Evaporation – Precipitation
Sea-surface salinity
Highest salinity in
open ocean occurs between 20-30 degrees N and S latitude
Greatest evaporation
Coincides with deserts
on land
Global sea-surface salinity
Seawater density
Isopycnals – lines of
constant density
Remember, density is controlled by
temperature & salinity
Density structure of the oceans
Dominated by temperature differences,
because salinity differences are
relatively minor
Atlantic
Ocean
sea-surface salinity
latitudinal
salinity transect
Main water
masses:
NADW – North Atlantic Deep Water
AABW – Antarctic Bottom Water
AIW – Antarctic Intermediate Water
The Rock Cycle and Seawater
Main
point: the primary salts in the ocean
have been derived from the weathering of igneous rocks